• Deal will double the doses available for immunization push
• Merck, Warp Speed strike pact on experimental treatment
Pfizer Inc. and partner BioNTech SE agreed to supply an additional 100 million doses of their Covid-19 vaccine to the U.S., as the country seeks to widen its immunization program and revive its economy.
The agreement brings the total number of doses to be delivered to the U.S. to 200 million, the companies said Wednesday in a statement. The drugmaker expects to deliver all the doses to U.S. vaccine and drug accelerator Operation Warp Speed by July 31.
Countries around the world are seeking supplies of vaccine they hope will allow the reopening of schools and businesses and the resumption of travel. The U.K. has also begun administering doses of the Pfizer-BioNTech shot, and European drug authorities cleared it for use on Monday.
Bloomberg said the U.S. has been working to expand supplies of the front-runner vaccine, in light of the drugmakers’ commitments to other countries. Earlier this month, the U.S. exercised an option to buy 100 million additional vaccine doses from Moderna Inc., doubling the number it has on order from that company to 200 million.
Like Pfizer’s vaccine, Moderna’s is a two-shot regimen. It doesn’t have to be stored at the same ultracold temperatures as the Pfizer shot.
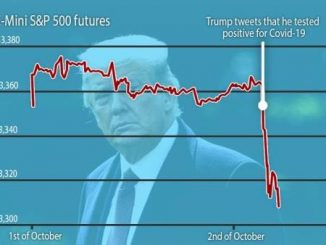
ALSO READ: Trump rejects $900bn Covid-19 pop relief package, calling it ‘a disgrace’
In July, the U.S. had agreed to pay $1.95 billion for an initial 100 million doses from Pfizer, with an option to buy 500 million more. The U.S. will also pay $1.95 billion for the new order, according to the statement.
Pfizer shares gained 0.7% in premarket trading in New York.
The U.S. also signed an agreement with Merck & Co. to supply doses of an experimental Covid drug that the company gained in its recent purchase of OncoImmune. The government will fund the development, manufacture and distribution of the drug, called CD24Fc, upon approval of an emergency-use authorization, Merck said in a statement.
Merck will receive about $356 million for 60,000 to 100,000 doses, according to the statement. The therapy is designed to tamp down inflammation caused by virus-induced damage to human cells, an underlying cause of some complications, and is expected to be used for treatment of severely ill Covid patients.
Merck agreed in November to buy OncoImmune with an upfront payment of $425 million in cash.